Chegg Which of the Following Compounds Are Soluble in Water
What ions are produced by each compound in aqueous solution. We review their content and use your feedback to keep the quality high.
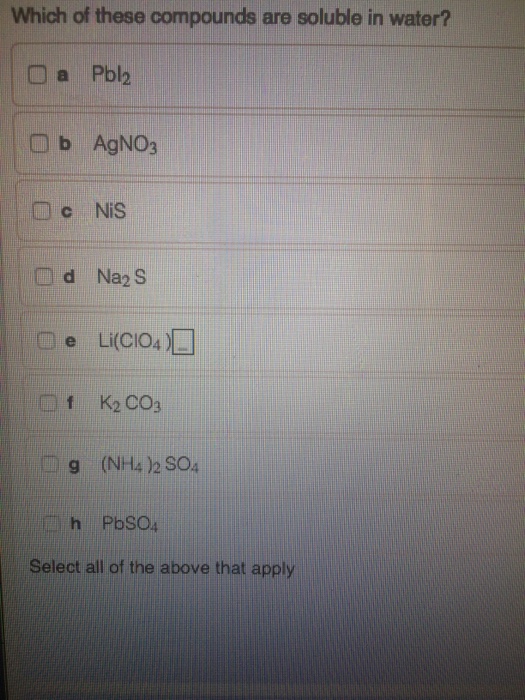
Solved Which Of These Compounds Are Soluble In Water Pbl 2 Chegg Com
Which one of the following compounds will be soluble in water.

. Metal oxides on the other hand are bases. View the full answer. Experts are tested by Chegg as specialists in their subject area.
A solution that has an osmotic pressure more than that. Common sodium potassium and ammonium compounds are soluble all nitrates are soluble common chlorides are soluble except those of silver and leadII common sulfates are soluble except for those of barium calcium and leadII common carbonates are. 234 know the general rules for predicting the solubility of ionic compounds in water.
Student Solutions Manual for WhittenDavisPeckStanleys Chemistry 9th Edition Edit edition Solutions for Chapter 3 Problem 105BYK. Two of these would be soluble. I 13 4 i 4 bIron sulfate FeSO4 FeSO4 ----------- Fe.
Based on the solubility rules which one of these compounds is soluble in water. Based on the solubility rules which one of these compounds is soluble in water. The result is an aqueous solution.
During a redox reaction the reducing agent becomes oxidized. Drag the appropriate items to their respective bins. What is Determine Whether Each Of The Following Compounds Is Soluble Or Insoluble Chegg.
All of the following compounds are soluble in water. Write the ions present in a solution of K3PO4. 3 Cl-.
Of these compounds the alcohol 1-propanol is most soluble. Common sodium potassium and ammonium compounds are soluble all nitrates are soluble common chlorides are soluble except those of silver and leadII common sulfates are soluble except for those of barium calcium and leadII common carbonates are. Metal oxides formally contain the O2 ion which reacts with water to give a pair of OH ions.
Reset Help Soluble Insoluble. AgNO 3 and AgClO 4. Click card to see definition.
234 know the general rules for predicting the solubility of ionic compounds in water. A Chromium chlorate III CrCl3--------- Cr3. Jul 21 2021 Double displacement reactions may be classified into several categories including counter-ion exchange alkylation neutralization acid-carbonate reactions aqueous The reaction between vinegar and baking.
Which of the following compounds is soluble in water. Click again to see term. In fact it is miscible.
Determine whether each of the following compounds is soluble or insoluble. Complete the following reaction and identify the Brønsted acidNaOHaq HClaq. View the full answer.
Formaldehyde acetaldehyde and acetone all are water soluble. Predict whether the following compounds are soluble or insoluble in water KNO3 CoBr2 AgNO3 AgBr BaSO4 NiCO3. Tap again to see term.
Zn s 2 HCl aq ZnCl2 aq H2 g TIP. Water is a polar solvent. In this single displacement reaction what substance is the reducing agent.
Because these compounds are only slightly soluble. 80 5 ratings Compounds given Ie SrCO3 SrSO4 PbBr2 are Insoluble in water. Tap card to see definition.
Determine Whether Each Of The Following Compounds Is Soluble Or Insoluble Chegg. Nonmetal oxides dissolve in water to form acids. The following compounds are water-soluble.
Only SrS is soluble in wat. CO2 dissolves in water to give carbonic acid SO3 gives sulfuric acid and P4O10 reacts with water to give phosphoric acid. Consider the water solubility of the following compounds of comparable size and molecular mass.
These are polar molecules and dissolve in polar solvent.
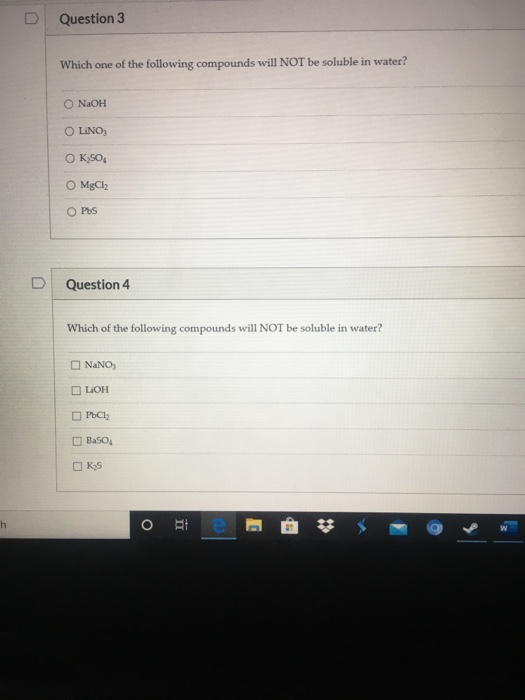
Solved D Question 3 Which One Of The Following Compounds Chegg Com
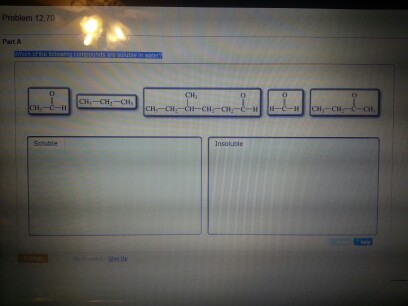
Solved Which Of The Following Compounds Are Soluble In Chegg Com
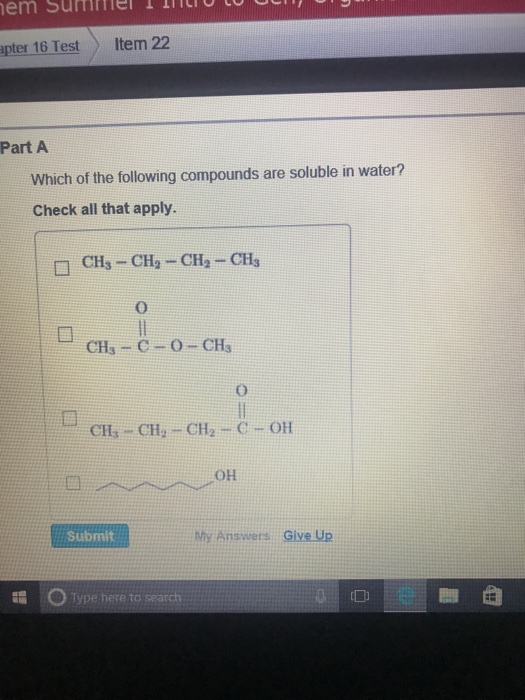
Solved Which Of The Following Compounds Are Soluble In Chegg Com
No comments for "Chegg Which of the Following Compounds Are Soluble in Water"
Post a Comment